Mohammed Alim Khan (1880-1944), Emir of Bukhara, taken in 1911. This is an early colour photograph taken by Sergei Mikhailovich Prokudin-Gorskii as part of his work to document the Russian Empire. Three black-and-white photographs were taken through red, green and blue filters. The three resulting images were projected through similar filters. Combined on the projection screen, they created a full-colour image.
When photographs were seen for the very first time, they were greeted with a sense of wonder. There was however also a sense of disappointment. People didn’t understand how a process could record a scene in such great detail but fail to record its colours. “The images produced by M. Daguerre are exquisitely correct, but gloomy-looking. They resemble moonlight pictures done in ink,” noted an editor of the Literary Gazette. The search immediately began for a means of capturing accurately not only the form but also the colours of nature. Many of the early pioneers, including Daguerre and Talbot, worked actively on this problem, and some had a measure of success. As early as 1840, the Englishman John Herschel (1792-1871) reportedly produced a primative colour image based on the interference principle (see Gabriel Lippmann below). Around 1850, Frenchmen Edmond Bequerel (1820-1891) and Claude Félix Abel Niépce de Saint-Victor (whose uncle had made what is generally credited as the first photographic image in 1826) and American Levi L. Hill (1816-1865) all reportedly produced colour images. However, none of these early efforts could be fixed reliably, and the images faded shortly after they were made. Photographers therefore began to add colour to their monochrome images by hand.
Hand Colouring
Photographers took to adding colour to the surface of their photographs with pigments, oils, watercolours and other substances. As the writer of A Guide to Painting Photographic Portraits noted in 1851:
“When the photographer has succeeded in obtaining a good likeness, it passes into the artist’s hands, who, with skill and colour, give to it a life-like and natural appearance.”
 Hand-coloured stereo daguerreotype of a young man in military uniform
Hand-coloured stereo daguerreotype of a young man in military uniform
Examples of this technique date from the very beginning of the medium, and it remained the most popular method of producing colour photographs for about 100 years. Whilst the Daguerreotype and Calotype were both independently invented in the late 1830s, it was the latter process that was the most easily coloured. The salt or albumen prints derived from this process could be coloured with watercolours or transparent oil paints and it proved to be a cheaper, simpler alternative to early colour processes. It also provided studio employment for miniature painters who had initially felt threatened by the emergence of photography.
In skilled hands, effects of great subtlety and beauty could be achieved, although colouring styles varied widely. Some colourists treated the image faintly - for example, a mild tinting of the cheeks. Others emphasized specific items of clothing, jewelry, or facial features. Some used transparent watercolours whilst others opted for heavy oils to cover the original image completely. Regardless of the approach, even at its very best hand-colouring remained an unsatisfactory means of recording colour; it could not reproduce the colours of nature exactly.
Whilst the matt-surfaced, uncoated Calotypes took colour easily, it was not easy to colour the non-absorbent surface of a metal-based daguerreotype. Produced on a polished silver surface of a copper plate, the Daguerreotype image consisted of tiny and delicate particles of a mercury-silver amalgam, easily brushed from the surface of the plate. Whilst the daguerreotype was hugely popular owing to the level of detail it could capture, the results with hand-colouring were generally quite subtle and subdued (sometimes no more than gold paint embellishment was used to highlight rings and other jewellery) and often quite unsatisfactory.
The popularity and commercial success of the Daguerreotype process only lasted for about twenty years and after 1860 the wet-collodion process took over, to be replaced in turn by gelatine dry-plates during the last quarter of a century. During that same period there was growing acceptance that “indirect” three-colour methods would eventually provide solutions to the photographic reproduction of colours.
Early Experiments
Thomas Young and Hermann von Helmholtz
Before colour could be reproduced, natural light and the existance of colour had to be clearly understood.
The scientific investigation of colour began in the 17th century. In 1666, Sir Isaac Newton (1643-1727) split sunlight with a prism to show that it was actually a combination of all colours which can be broken out into a colour ‘spectrum’ (Latin for “appearance”). He divided the spectrum into seven sections — red, orange, yellow, green, blue, indigo, and violet — in keeping with the Greek sophists, to connect the colours to days of the week, musical notes, and the known objects of the solar system. In reality it is hard to distinguish indigo sufficiently from either blue or violet and therefore it is often omitted from the modern spectrum. This experiment correctly identified that different colours of light move at different speeds (wavelengths - but then incorrectly described as particles), which was a vital foundational concept for understanding our perception of colour.
The theory of colour vision was developed and propounded by Thomas Young (1773-1829) in the 1802, later elaborated by Hermann von Helmholtz (1821-1894) in 1850. The Young–Helmholtz theory (also known as the trichromatic theory) is a theory of trichromatic colour vision – the manner in which the visual system gives rise to the phenomenological experience of colour. In 1802, Young reasoned that, since all colours could be matched by an appropriate mixture of red, green and blue light, the eye must contain three kinds of photoreceptors (now known as cone cells) responding to these colours only, and therefore all other colours found in the spectrum will be seen as a mixture of those three primary sensations.
 Hermann von Helmholtz on photoreceptors
Hermann von Helmholtz on photoreceptors
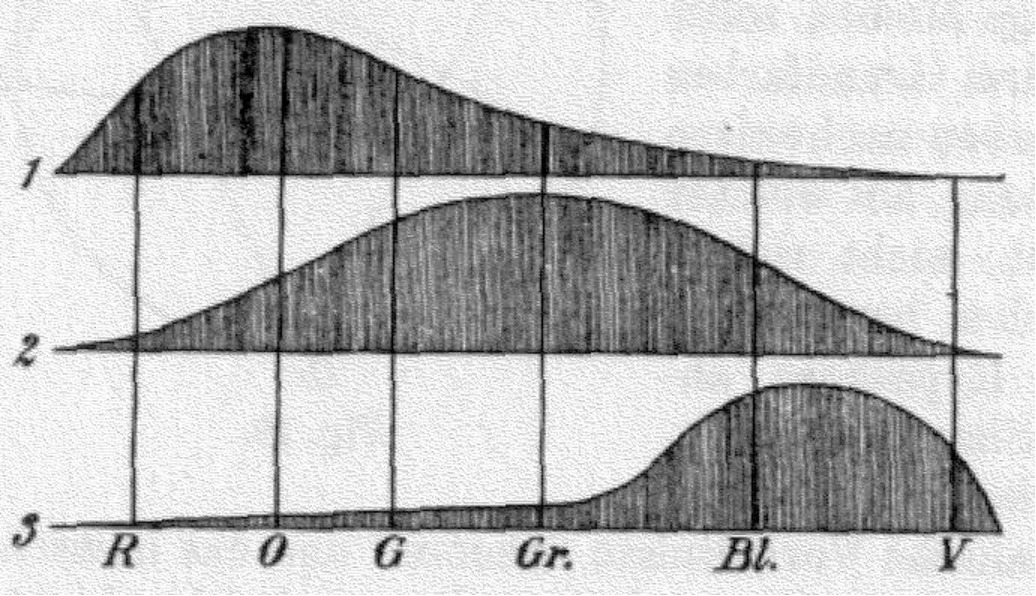
Hermann von Helmholtz developed the theory further in 1850: hypothesising that the three types of cone photoreceptors could be classified as ‘short-preferring’ (violet), ‘middle-preferring’ (green), and ’long-preferring’ (red), with different but overlapping responses to different wavelengths of visible light. The relative strengths of the signals detected by the three types of cones are interpreted by the brain as a visible colour.
James Maxwell
In 1861, Scottish physicist James Clerk Maxwell (1831-1879)conducted an experiment to show that all colours can be made by an appropriate mixture of red, green and blue light.
Maxwell, with the help of photographer Thomas Sutton, made three lantern slides of a tartan ribbon using the wet collodion process through red, green and blue filters. Using three separate magic lanterns - each equipped with a filter of the same colour the images had been made with - he then projected them onto a screen. When the three images were superimposed together on the screen, they combined to make a full-colour image which was a recognisable reproduction of the original. This process is called Trichromatic Photography and can still be practiced today.
 Thomas Sutton / James Clerk Maxwell, Tartan ribbon, 1861. Vivex print (1937)
Thomas Sutton / James Clerk Maxwell, Tartan ribbon, 1861. Vivex print (1937)
The principle established by Maxwell’s experiment was that of additive colour photography. Starting with a blank screen he added together three images in the additive primary colours (red, green and blue) to reproduce the original colours. Subtractive colour formation was originally proposed by Louis Ducos du Hauron and fellow Frenchman Charles Cros, who worked independently of each other but announced their findings at about the same time in the late 1860s. Whilst Ducos du Hauron concentrated on practical applications, Cros focused primarily on theory.
Louis Ducos Du Hauron
Louis Ducos du Hauron (1837-1920) was the first to succeed in producing stable colour images on paper using the same colour seperation technique as Maxwell. In 1862 he submitted a paper on the reproduction of colour through photography to the Academy of Sciences in Paris, but his work was rejected. Undeterred by the negative comments, he continued his research on colour. In 1868 he applied for a patent in which he described how to make sets of seperation negatives and several methods for obtaining colour photographs through subtractive means.
He proposed several methods for producing colour photographs using a combination of three colours. In 1869 Hauron published “Les Couleurs en photographie, solution du problème”, in which he described both a simpler design of the three-plate ‘one-shot’ colour camera and also a ‘mosaic’ filter additive screenplate. He correctly anticipated many of the theoretical frameworks for making analogue colour photographs.
a) ‘One-shot colour’ - Hauron theorised that the additive process could be applied in a manner that did not require the complicated separation process that Maxwell devised by combining coloured pigments instead of light. Three black-and-white negatives, taken through red, green and blue filters, could be used to make three separately dyed images which combined to give a coloured photograph. Using the black-and-white channel negatives, he made positive images then stripped off the positive emulsion and applied pigments using gelatin coatings. For the pigments, Ducos du Hauron used the complements of the filters through which he had shot the negatives. The gelatin absorbed the pigment of each in proportion to the density of the negative. Hauron shared examples of colour prints made using this method and his English patent of 1876 gave a clear description of what we now call the subtractive process.
 The town of Agen in Aquitaine, France, and the twelfth-century cathedral of St Caprais. Taken in 1877 by Hauron it is the first surviving colour photograph of an outdoor scene and is a tricolour carbon transparency mounted on paper.
The town of Agen in Aquitaine, France, and the twelfth-century cathedral of St Caprais. Taken in 1877 by Hauron it is the first surviving colour photograph of an outdoor scene and is a tricolour carbon transparency mounted on paper.
b) ‘Mosaic colour’ - Du Hauron speculated that a screen ruled with fine lines in the three primary colours would act as a filter to produce a colour photograph with a single exposure instead of the theoretical three needed in Maxwell’s experiment. He photographed each scene through green, orange, and violet filters, then printed his three exposures on thin sheets of bichromated gelatin containing carbon pigments of red, blue, and yellow. When the three positives (transparencies) were superimposed, a full-colour photograph resulted, pointing the way for future single-colour exposures.
Charles Cros
Around the same time Charles Cros published a paper in “Les Mondes” with the title: “Solution generale du problem de la photographie des couleurs”, independently demonstrated how colour images could be made using three-colour separation negatives.
Later in 1879 he published details of a practical design of a ‘Chromometre’ - a device for measuring and analysing colours by mixing red, green and blue light, but also suitable for adaption to view sets of three positive transparencies made from negatives exposed through red, green and violet filters.
However Cros was a theoretician rather than a practical inventor and therefore had neither the means nor the desire to commercially exploit his ideas. These early suggestions from both Cros, Hauron and others were of very limited practical value anyway since the photo-sensitive materials available at the time would respond only to blue light. Practical application had to wait until photographic materials sensitive to the whole spectrum had been discovered. Improvements were made in this department towards the end of the nineteeth century, but it wasn’t until 1906 that the first fully panchromatic plates, sensitive equally to all colours, were sold by Wratten and Wainwright of London.
It should also be remembered that throughout the latter half of the nineteenth century, stereoscopic photographs were extremely popular and to a large extent satisfied public interest in photography. Whilst Du Hauron and Cros made it clear they knew how to produce colour prints from separation negatives in the 1870s, the process was extremely difficult. There was therefore limited public appetite for their ideas.
Gabriel Lippmann
Not all early colour photography research concentrated on additive and subtractive principles. Some of the most promising work was based on interference principles, which produced colour through the chemical response of a silver chloride emulsion to reflected light waves, much the same way colour is produced on oil slicks or in mother-of-pearl when viewed at a certain angle.
French physicist and Nobel Prize winner, Gabriel Lippmann, came close to success with his interference efforts. The process he introduced in 1891 produced remarkably natural colours, but he was not able to solve many of the problems that had plagued earlier interference experiments, including long exposures and awkward viewing methods.
Additive Colour Processes
The first commercial application of the additive process was the work of an American, Frederic E. Ives, an American painter and prolific inventor of colour photographic processes throughout his life. As early as 1888 he was attempting to develop a colour printing system, but later focused his attention on the design on additive viewing devices.
The Kromogram
In 1895, Ives devised a system similar to Hauron’s Chromoscope, where colour seperations were created when light entered his camera and bounced off various reflective surfaces into three seperate channels through either a blue, green, or red filter to create three separate black-and-white photographs of the subject. By using an external mirror system, stereoscopic pairs of colour separations could be taken with the same camera.
 Kromskop stereo viewer in wooden box with slides
Kromskop stereo viewer in wooden box with slides
The negatives were printed on glass as transparencies and placed in a special viewer, called a Kromskop. Mirrors in the Kromskop superimposed the images on the three transparencies and a second set of filters restored the colours. He later introduced the Projection Kromskop for colour lantern slides.
Whilst the quality of colour in the Kromograms was praised, the system was prohibitively expensive and ultimately too bulky and complex to be a commercial success. Nevertheless, versions of his processes (variously called three-colour or colour-seperation process) remained in use well into the twentieth century, primarily because they were well suited for making photoengravings. A last notable refinement, the Devin One-Shot, was introduced as late as the 1940s. The original Kromogram, was however discontinued shortly after the 1907 introduction of the Autochrome process, which was simple to use and required no special equipment.
 Kromogram stereo slide with final image enlarged
Kromogram stereo slide with final image enlarged
The Joly Process
Additive screen plate processes represented the next generation of colour photography. Instead of making three separate exposures through red, green and blue filters, a simpler approach was to make just one exposure through a filter that combined all three primary colours.
The first process to use this method was devised by Irishman Dr John Joly in 1893. His method followed the process that Du Hauron previously outlined in 1869, “a paper whose surface is simply covered with alternating lines of red, yellow and blue… At a distance these lines merge into a single colour.” His camera exposed a single plate of black-and-white film through a “taking screen” of microscopic lines of blue, green, and red dyes (A yellow filter was also added over the camera lens to correct for the plate’s excessive blue sensitivity). The resulting black-and-white negative was printed onto another plate to produce a positive sheet of film. The positive reproduced the colour of the original subject when seen with a white light through a “viewing screen” that lined up with the original taking screen.
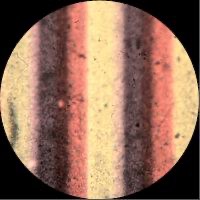
Joly covered a glass plate with very fine red, green and blue lines (<0.1mm wide) in order to create a three-coloured filter screen.

The Joly process was introduced commercially in 1895 and remained on the market for a few years. However, like so many early colour methods, Joly’s screen process was awkward and slow. It was difficult to line up the taking and viewing screens with precesion and the limited colour sensitivity of the plates and the width of the lines across the image meant the results were not very successful.
Still, the Joly screen-plate process was widely imitated and improved upon, using both dot and line configurations in the filter screens, but commercial success for most of these inventions was limited.
The Autochome
The first properly usable and commercially successful screen process - the Autochrome - was invented early in the twentieth century by two French brothers, Auguste and Louis Lumière. They had been experimenting with colour photography since the 1890s, and published their first article on the subject in 1895. In 1904 they gave the first presentation of their process to the French Academy of Science, and by 1907 they had begun to produce autochrome plates commercially.
News of their discovery soon spread, the response was enthusiastic and examples of the new plates were in high demand. Their process went on to dominate colour photography during the early years of the century.


“The possibilities of the process seem to be unlimited… soon the world will be color-mad, and Lumière will be responsible” Alfred Stieglitz, photographer (July 1907)
Their success was due to the ingenuity of the process, the comparative perfection of the results obtained, and the commercial strategies of the Lumière brothers. Realising there was no need to keep the filter screen separate from the photographic emulsion, the Lumières combined both screen and emulsion on the same glass support. It used a mosaic screen composed of starch grains dyed red-orange, green and blue-violet in front of a fine-grain panchromatic emulsion.
Manufacturing autochrome plates was a complex process. First, pulverised starch grains were passed through a sieve to isolate individual grains between 10–15 microns (0.010 - 0.015 mm) in diameter. Many different types of starch were tried, but potato starch gave the best results. These microscopic grains were then dyed, mixed and spread over a glass plate, and coated with a sticky varnish. Loose grains were removed by brushing.
The plate then passed under a roller to flatten the grains and narrow any gaps between them, any remaining gaps were filled with fine black charcoal powder. The plate was then varnished to make it waterproof.
The final plate was a three-coloured filter screen with approximately four million transparent starch grains per square inch, each grain effectively acting as a coloured filter.
Autochrome plates were simple to use. They required no special apparatus and photographers were able to use their existing cameras. However, like all early colour processes exposure times were very long - approximately 30 times that of conventional plates. Even in bright sunshine, an exposure of at least one second was needed, and in cloudy weather this could be increased to 10 seconds or more. Even in a well-lit studio, portraits could require an exposure of as long as 30 seconds. This was due to the inherent slowness of the emulsion, the added density of the colour filtering starch grains, and the need for a filter on the lense to help achieve accurate colour.
Following exposure, autochrome plates were reversal-processed to produce a positive image. When viewed by white light passing through the plate, the millions of tiny red, green and blue-violet grains combined to give a full-colour photograph, accurately reproducing the colours of the original subject.
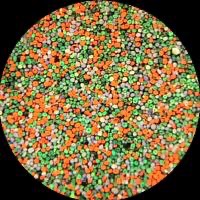
In theory, the grains were mixed and distributed randomly on the surface of the plate. In practice, however, mathematical probability meant some grouping of grains of the same colour was inevitable. While individual grains are invisible to the naked eye, these groups of clumps are visible - appearing as random blotches of blue, green and red. The filters also sometimes overlapped, with reds, merging into greens and greens into blues. The effect diluted the quality of the colour, making it appear less accurate and bright, and softened the image, which often looked slightly out of focus. Whilst the process was flawed, photographers embraced these imperfections and appreciated autochrome’s distinctive beauty, comparing the output with the work of Impressionist and Pointillist painters.
 Autochrome group portrait of children celebrating a ‘May Queen’ gala day, unknown photographer, c.1910
Autochrome group portrait of children celebrating a ‘May Queen’ gala day, unknown photographer, c.1910
By 1913, the Lumière factory in Lyon was producing 6,000 autochrome plates every day. The commercial success of the process prompted the appearance of many other colour processes based on the concept of screens made up of microscopic colour filters. These screens used either a random grain pattern or, more commonly, different geometric patterns of lines and squares.
Dufaycolor
Whilst many of the photographic processes introduced to the market at this point are now long forgotten, one remained popular for years - the Dufaycolor process devised by French inventor Louis Dufay.
Dufaycolor (1910) used a flexible film base rather than a glass plate and first appeared in 1932 as a 16mm cine film, followed in 1935 by a roll film version. It was an additive combined screen process that employed a very fine geometric screen made up of red lines alternating with rows of green and blue rectangles printed on a non-inflammable film base coated with collodion. Colour reproduction was good, and it was comparatively fast—although only one-third of the speed of available black-and-white film.
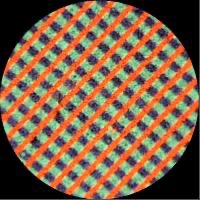
Whereas autochromes appealed to keen photographers who liked to do their own processing, Dufaycolor was aimed at the everyday ‘snapshot’ market. A processing service which returned finished transparencies, mounted and ready for viewing, opened up colour photography to a whole new class of photographers.
Dufaycolor, the last of the additive screen processes, remained on the market up to the 1950s. It could not survive the competition of the modern screen-less colour processes. The principle has, however, been revised in digital screens, where the picture is produced in the form of minute red, green and blue dots ’lit’ by electronic signals.
 A Dufaycolor colour transparency, unknown photographer, c.1950
A Dufaycolor colour transparency, unknown photographer, c.1950
 A Dufaycolor colour transparency of the River Thames and the Houses of Parliament, London, unknown photographer, c.1945
A Dufaycolor colour transparency of the River Thames and the Houses of Parliament, London, unknown photographer, c.1945
Other notable products at the time included Agfa Colour (1916), which packed coloured grains in a tighter pattern; and Finlay Colour (patented in 1906 but not marketed until 1929), which used independent taking and viewing screens to accomodate a variety of black-and-white emulsions.
Subtractive Colour Processes
The processes considered so far have reproduced colour by adding together three light impressions coloured red, green and blue, either by projection, other forms of optical combination, or by the use of colour screens. They relied on the principle of blocking out light to capture the three primary layers and adding them back together on viewing.
These processes had two main disadvantages:
• They relied on the use of filters to block out light, which resulted in long exposure times and very dense transparencies
• The colour photographs made using these processes could only be viewed by transmitted white light - i.e. by projection or by using special viewing devices
This additive principle can be compared to the techniques of a modeller, who starts with an empty stand and adds clay to it until the whole creation is complete. There was, however, an alternative method of reproducing colour photographically - ‘subtractive’ colour synthesis - comparable to a sculptor taking a block of wood or stone and carving away the material that is not needed. The photographic equivalent begins with white light, in which all the colours are present, and removes those colours we do not want as to reproduce the original scene.
The original theory for subtractive colour reproduction can also be traced back to Louis Ducos du Hauron, who explained the method in his book “Les couleurs en photographie, solution du problème” (1869). Du Hauron proposed that colour separation negatives should be used to produce three positive images, which would then be dyed the complementary colours of cyan (bluegreen), magenta (blue-red) and yellow. This led du Hauron to obtain a French patent for the first “one-shot” camera designed specifically for subtractive colour photography in 1874, following up with an English patent two years later.
Each of these complementary colours absorbs - or subtracts (hence the name) - one of the primary colours. Cyan absorbs red light, reflecting a mixture of blue and green light. A cyan image, therefore, performs the same function as the red filter used in an additive process. Similarly, magenta absorbs green light and yellow absorbs blue light. By accurately superimposing these three complementary colours, all other colours can be reproduced. The colour in subtractive processes comes from dyes or pigments rather than coloured filters.
With subtractive colour, white, for example, is represented by clear glass or white paper rather than by light passing through three filters. This means that subtractive processes are much less wasteful of light.
More importantly, they work with reflected rather than transmitted light, meaning they can be used to produce colour photographs on paper. The development of subtractive colour processes followed two distinct paths. Firstly, the design of specialised cameras - for taking sets of colour separation negatives - and secondly, the search for practical methods of making and superimposing three positive images in the complementary colours.
When taking colour separation negatives of stationary still life subjects a conventional camera could be used. The colour filter simply needed to be changed after each exposure. This procedure could be made simpler through the use of a ‘repeating back’, a moving part of the camera which allowed filters of different colours to drop into place, and companies such as Sanger Shepherd began to manufacture these attachments.
A number of devices of this sort were marketed. The simplest type were long plateholders, fitted with three filters, which the photographer would manually slide along the camera back in three steps. The most complex were fitted with clockwork motors, enabling three negatives to be exposed in rapid succession in as little as two or three seconds.
 Gandolfi extending bellows camera fitted with a repeating back, 1930
Gandolfi extending bellows camera fitted with a repeating back, 1930
When photographing subjects with limited movement (e.g. portraits) even automatic repeating backs were not fast enough. For these, a camera that could expose all three negatives simultaneously was needed.
Over the years, many designs for such ‘one-shot’ cameras were patented, and a number were produced commercially. These used various arrangements of mirrors and prisms to split the light entering the camera into three separate beams, each of which went to a black-and-white plate fitted with a different coloured filter. Among the most successful designs were the Jos-Pe, Bermpohl, Klein and Mirkut cameras. This method was also employed for the Lumiere Brothers’ Trichromie (a.k.a. Trichrome) process.
 Jos-Pe tri-colour camera, c.1925
Jos-Pe tri-colour camera, c.1925
Carbon Printing
Obtaining satisfactory negatives was just the first stage. These negatives then needed to be converted into positive images in the complementary colours of cyan, magenta and yellow.
Several different methods were used to obtain these images, the most popular being variations of the carbon process. These used sheets of carbon tissue, consisting of a gelatine coating, containing pigment, on a paper base. The tissue was sensitised before use by soaking it in potassium bichromate. Potassium bichromate hardens when exposed to light so, after exposure in contact with a negative, the areas of unhardened gelatine could be washed away to reveal an image.
Tissues could be produced using pigments of any colour; images on cyan, magenta and yellow tissues were then superimposed to produce subtractive colour prints.
A variant of the carbon process was the Trichrome Carbro process. It used a set of bromide prints made from separation negatives to make the necessary yellow, magenta and cyan pigment images on tissue for transfer in sequence on to a paper base. The process was first developed during the 1890s but made popular by the Autotype Company of Ealing during the 1920s and 1930s.
 An Ozobrome print (a variation of the three-colour Carbro process), unknown photographer, c.1930
An Ozobrome print (a variation of the three-colour Carbro process), unknown photographer, c.1930
While processes such as Carbro were available for amateur photographers to use, tissue assembly techniques were difficult and complex - reserved for only the most dedicated hobbyists. Amateurs preferred to use additive processes such as the autochrome process and Dufaycolor.
The Vivex Process
During the 1930s, commercial colour photography became increasingly important. For professional colour printing, at this time, one process reigned supreme: Vivex.
Invented in 1928 by Dr Douglas A. Spencer, who later went on to become Managing Director of Kodak, Vivex was a modification of the Trichrome Carbro process in which sheets of cellophane were used as temporary supports for the pigment images. Any minor problems with the image could be corrected manually by stretching or squeezing the cellophane to ensure perfect superimposition.
To exploit the Vivex process, a company called Colour Photographs (British & Foreign) Ltd. was formed with a factory in Willesden, North London. This was the first laboratory to offer a colour print making service to professional photographers. Such was the popularity of the Vivex process that it has been estimated that over 90% of all the colour prints made in Britain in the 1930s were produced using it.



The Tripack System
Subtractive colour processes, such as Vivex, required colour separation negatives to be made on three separate photographic plates. However, if all three could be combined into a single unit - or tripack - there would be no need for specialised colour cameras or repeating backs fitted with filters.
It was the invention of tripacks that paved the way for the development of ‘modern’ colour processes such as Kodachrome. The basic idea of the tripack system was to construct a multi-layer unit, where each plate was coated with an emulsion sensitive to one of the primary colours. Light would pass through the first plate in order to reach the second emulsion layer and, in turn, pass through that plate to register on the third emulsion.
The first practical tripack system was introduced by Frederic Ives in 1916. His ‘Hiblock’ tripack consisted of a sheet of film sandwiched between two glass plates. The top plate was blue-sensitive, the film was green-sensitive and the bottom plate was sensitive to red light. After exposure, the three layers were separated for processing; the negatives were then treated as conventional separation negatives.
The tripack system was quicker than previous colour photography processes, but resulted in blurry negatives. The solution to this problem was to coat all three emulsions onto the same glass or film support. This was called an ‘integral tripack’. Since it was physically impossible to separate the layers, each had to be capable of being chemically processed in isolation so as to produce an image in cyan, magenta or yellow. In 1912, German chemist Rudolph Fischer had patented a proposal to use what later became known as ‘colour couplers’ - substances that react with chemicals formed during development to create coloured dyes. Fischer suggested that colour couplers for producing cyan, magenta and yellow dyes should be incorporated into the appropriate layers of an integral tripack so that coloured images would be formed during development. The result would be a full colour photographic image.
Fischer’s process could be used in any camera, which was significant since most early processes required the use of dedicated equipment. Unfortunately, the colour couplers Fischer used tended to disperse between emulsion layers during processing. Fischer’s theory, however, was sound, and his work was to form the basis of the research that led to the first commercially successful integral tripack system - Kodachrome.
Kodachrome
Kodachrome was the invention of Leopold Mannes and Leopold Godowsky. Both earned their living as professional musicians while spending their spare time experimenting with colour photography. Despite their best efforts, there came a point when they were unable to progress without outside support.
This support was to come from Dr C.E. Kenneth Mees, director of the Eastman Kodak research laboratories in Rochester, New York. In 1922, Mees met with Mannes and Godowsky and, impressed with the quality of their work, agreed to supply them with the materials they needed to continue their research. At this point, they were working on a two-colour subtractive system for colour photography but, after reading about Fischer’s work with colour couplers, they decided to abandon their previous methods and concentrate on developing a three-colour multi-layer film system. In 1931, the two Leopolds gave up their musical careers to work full-time in the Kodak research laboratories where, with the help of Eastman Kodak’s enormous resources, they made rapid progress.
 Kodachrome colour landscape photograph, unknown photographer, c.1945
Kodachrome colour landscape photograph, unknown photographer, c.1945
 Kodachrome colour transparency of man operating paint mixing machine, unknown photographer, c.1945
Kodachrome colour transparency of man operating paint mixing machine, unknown photographer, c.1945
Like Fischer, Mannes and Godowsky had great difficulty in preventing the coloured dyes spreading between the emulsion layers. They overcame this by putting the colour couplers in the developer rather than the emulsion. As a result, Kodachrome is in effect a three layered black-and-white film with different colour sensitivities to which coloured dyes are added during processing. The top layer is sensitive to blue light, the middle layer to green and the bottom layer to red. There is also a yellow filter layer positioned below the blue to keep blue light from affecting the bottom layers but this disappears when the film is processed.
Each black-and-white emulsion layer records light in proportions to the original colours of the subject. The blue-sensitive layer, for example, receives the most exposure from blue parts of the subject. In the first developing stage, the film turns into the equivialent of three seperate black-and-white negatives, with silver density deposited on each layer according to the amount of exposure each area of the layer has received. Thus, a blue sweater records with greatest density on the film’s blue layer. The second development reverses the image to create a positive and introduces dye in each layer complementary to that layer’s original sensitivity (again in proportion to the original exposure). The overlapping complementary dyes form the image colour. Subsequent processing steps include beaching and fixing to remove the silver.
Kodachrome processing was therefore extremely complex and involved repeated development, dyeing and selective bleaching for each layer. In total it required at least 28 different stages that could only be carried out in laboratory conditions and photographers were unable to process their own film; they had to send it back to the Eastman Kodak laboratories in Rochester, NY. It was, however, a landmark invention, capable of producing high-quality colour images with sharpness and colour fidelity. On 15 April 1935, the first Kodachrome film went on sale for use in 16mm cine cameras. 35mm Kodachrome film was available on the American market in 1936, and the first supplies reached the UK in 1937. Improved versions remained the standard colour film for decades before being slowly discountined in the early 2000s, unable to compete with the rise of digital photography.
 Print advertisement for Kodachrome Film, 1941
Print advertisement for Kodachrome Film, 1941
For 35mm Kodachrome users, Kodak introduced 2"x2" cardboard Koda Slide Mounts in 1939. These were attached to individual film exposures at the processing lab so the results could be projected for viewing. In 1941, Kodak introduced its first colour printing service with Minicolor prints for 35mm and roll film cameras. Minicolor prints were made on an apalized white cellulose acetate base, with a feel similar to modern resin-coated papers. That same year, Kodak introduced Kotavachrome prints in larger sizes for professionals using Kodachrome in sheet form.
Agfacolor-Neu
 Agfacolor glass transparency of flowers in a vase, unknown photographer, 1930s
Agfacolor glass transparency of flowers in a vase, unknown photographer, 1930s
In 1936, Agfa, a German company, also announced a multi-layer colour film. Agfa had been making additive colour plates since 1916, so they called their colour film Agfacolor-Neu - ‘new’ to indicate that it was completely different from any earlier products. Agfacolor-Neu was the first commercial process to follow Rudolph Fischer’s theory of using colour couplers.
Agfa’s research chemists discovered a way of anchoring couplers in the individual emulsion layers to create the first chromogenic colour film. In the chromogenic process, the colour is formed, instead of added, during development. As the silver density builds, chemical by-products activate the couplers and release dyes in proportion to the silver build up on each emulsion layer. Subsequent bleaching and fixing remove the silver, leaving only the colour image.
They overcame the problem of migrating colour couplers by making their molecules very big. In this manner, they would mix easily with the liquid emulsion during the manufacturing of the film. Once the gelatin that bound them together had set, the colour coupler molecules were trapped in the tiny spaces of the gelatin and unable to move. This was the first three-layer, subtractive color reversal film that had the colour couplers built into the emulsion layers themselves and employed a single developer to make the positive image.
The overall solution made Agfacolor film much easier to process. Unlike Kodachrome, it could even be done by the user at home. After the Second World War, the details of Agfa’s research became freely available, and other companies - such as Ferraniacolor and Gevacolor - colour film based on the same principle. Whilst early chromogenic colour and image quality were not on par with Kodachrome quality, this changed over the years and chromogenic films came to dominate the market. With the perfection of dye-based multi-layer colour films such as Kodachrome and Agfacolor-Neu, a new era of colour photography had dawned.
Chromogenic Negative Film
The earliest negative films based on subrtractive colour principles actually came before Kodachrome. In 1928, ‘Color Snapshots,’ an English company, introduced ‘Colorsnap’ - a product based somewhat on Ducos Du Hauron’s concepts - but the company went bankrupt soon after. Agfa also licensed the product and produced a similar film. Agfa brought out a colour negative film in 1939 from which positive colour prints could be made directly on a special companion paper, but it was not commercialised. Kodak followed suit in 1942 with Kodacolor, which is considered to be the first marketable subtractive colour negative film that completely solved the problem of the colour couplers migrating from layer to layer in the emulsion. Colour negative films, such as Kodacolor, overcame the limitation of reversal films, where each image was one-of-a-kind, since any number of positive prints could be made from a negative.
The processing method created for Kodacolor is, with many improvements, the basis for all colour negative film processes utilised today. In this process a single developer produces a negative silver image and a corresponding dye image in all three layers of the emulsion at the same time. Bleach is used to remove all the silver, leaving only the dye. The film is fixed, washed, and dried, which completes the process.
When making prints from a chromogenic negative with the subtractive method, the light is filtered in the enlarger before it reaches the negative. Correct colour balance can thus be achieved in a single exposure. One of the three dye layers in the negative is usually left unfiltered. Printing is simplified because it is not necessary to use more than two filters at one time to make a print.
Negative film was especially popular with amateur photographers. It produced better-quality prints than transparency-to-print processes such as Minicolor. It also had more exposure latitude than transparency film, allowing colour photographs to be made using simple box cameras with rudimentary exposure controls. Early colour negative films were also faster (more sensitive to light) than early transparency films. When Kodacolor was introduced, it had a film speed equivalent to ISO 32, almost four times faster than the original Kodachrome.
Modern Film
Other, more complicated methods of making colour prints were also developed for professional photographers. Kodak’s wash-off relief process (1935) and dye-transfer process (1945) were related processes that took Ducos du Hauron’s discoveries several steps further. Both formed colour by absorbing dyes from a series of gelatin-relief images - a highly complex and time-intensive matter, but one that resulted in excellent colour fidelity and outstanding image stability.
Bela Gaspar, a Hungarian chemist, introduced the first successful silver dye-bleach colour process in 1934. This subtractive method produced colour by bleaching out unnecessary dyes already contained in the emulsion rather than activating or adding them. Originally released as a motion picture film, Gasparcolor was the forerunner of Ilfochrome, a popular modern process introduced in 1963 as Cibachrome.
Most modern films and papers are based on processes from the 1930s, but the materials have improved enormously. The colour is far more accurate, processing is quicker and less fussy, emulsions are much faster and images are razor sharp. Processing has also been universally standardised. For example, you can process various-speed transaprency films by Agfa, Fuji and Kodak (except for Kodachrome) in the same tank for the same amount of time.
In addition, there are now dozens of different colour films and papers, offering a wide range of colour palettes, speeds and other characteristics. Most have much-improved stability characteristics; when properly handled and stored, they last much longer than early films and papers, without fading or otherwise deteriorating.
Polaroid
Probably the most important colour process introduced after chromogenic materials came from Polaroid. Edwin Land, who founded the company, started his business by developing and selling polarizing filters. Land’s passion was vision research, and in 1947 he introduced the first instant photogrpahy system. In 1963, Polaroid added a colour version called Polacolor.
Land’s process was based on diffusion transfer, a technology used in early photocopiers. The film contains a negative and a positive receiving sheet to make the print. After exposure, a roller in the camera (or a seperate film holder) presses the sheets together for the image transfer, during which silver dyes migrate to the positive receiving sheet. After a couple of minutes, you can peel the negative from the positive, disgard the negative and keep the positive image.
In 1972, Polaroid introduced SX-70, a self-developing film that didn’t need to be peeled. In 1983, the company released several instant 35mm films, including one that was based on old-fashioned additive screen technology to form colour.
In the 1960s, NASA developed methods of digital imaging for recording and transmitting images of the planets from spacecraft. Digital cameras use electronic sensors, which break the image down into tiny picture elements (called pixels). In this form, an image can be stored in a computer, then seamlessly retouched, altered, and combined with other images and text. It can also be transmitted almost instantenously to other locations through telephone lines or microwave signals. In 1981, Sony introduced the Mvica, which was the first electronic camera for consumers. Using analog rather than digital signals, the Mavica recorded the image and stored it on a 2-inch disk.
Ilfochrome
The method by which Ilfochrome materials form colour is called silver dye-bleach. It differs markedly from chromogenic colour, the method used by almost all other colour films and papers.
The silver dye-bleach system uses an emulsion with three basic dye layers of complementary colours built in (one each of cyan, magenta, and yellow). During the processesing, the dyes that aren’t needed are bleached away, leaving the desired positive colour image. As with chromogenic processing, which produces its dyes during development, silver plays an intermediary roles. Each emulsion layer contains silver halides in addition to a dye. Each silver layer is sensitive to one of the primary additive colours, (blue, green, or red), which is linked to the appropriate complementary dye. Thus, the blue-sensitive layer contains yellow dye, the green-sensitive layer contains magenta dye, and the red-sensitive layer contains cyan dye.
Upon exposure, the silver in each layer responds to the amount of blue, green and red light in receives from the projected transparency. The red-sensitve layer, for example, is highly affected by red light (and to a lesser degree magenta and yellow, the colours next to it on the colour wheel); it is unaffected by cyan.
During development, the exposed silver in each layer is converted to a black metallic silver negative image (similar to what happens during black-and-white development). The amount of silver that builds up depends on the layer’s colour sensitivity. Thus, in the red-sensitive layer, a lot of density will build up in the areas where red light hits (magenta and yellow light will produce proportionallly less density, and cyan will produce none).
Bleaching, the next step, removes colour dyes in proportion to the silver density. Areas with alot of density are bleached more heavily, leaving dyes that match the colour of the transaprency when the three layers are superimposed.
Bleaching also converts all the silver (developed and undeveloped) into silver halide for removal in the fixer, which is the final critical step.
